Saturday, November 10, 2007
The Art of Mineral Separation
When many people in the geochronology/thermochronology community talk about new gadgets and gizmos on their wishlist they seem to focus almost entirely on the sample analysis side of things; particularly multi-collector noble gas mass spectrometers and various high end lasers. I'll admit, these machines are impressive and could be potentially very exciting, but if I had a pot of money to spend to increase the quality and quantity of the data my labs produce, they would not be the first thing I'd look into.
In a broad sense, doing meaningful thermochronology requires 4 basic techniques.
1. You must be able to identify tectonic and/or geochemical problems that can be be at least partly addressed thermochronologically. This starts with the big picture, but includes consideration of available lithologies and access to the necessary samples.
2. You then need to collect and process the samples. This means turning a 5 kg sack of rocks collected carefully in a very specific location to individual mineral separates ready for your lab.
3. Once you have mineral separates, you need to analyze them in a lab. Although actually collecting data in a lab is fairly trivial, running a lab well enough to insure that your data actually means something is not.
4. Once you have the data, you need to interpret it, again in order to answer the original tectonic and/or geochemical problem you set out to solve in the first place.
Steps 1 and 4 are probably the most complex, and in my opinion are the hardest skills to develop. To design and interpret good projects you need a strong background in basic geology and need to consult all of the experts that relate to the study. In my own work I need enough background to understand what the petrologists, sedimentologists, geophysicists, geomorphologists, structural geologists, and geochemists think. This requirement is not unique to thermochronologists. I'd argue that any geologist who considers tectonic questions is necessarily broad in scope. So steps 1 and 4 I see as general considerations for any earth science study.
Step 3 receives a great deal of attention. I've been a thermchronologist for less than a decade, but even in that time the number of new and expensive machines and techniques has ballooned. I've been involved with building and maintaining labs, and therefore have paid a lot of attention to these advances. As I've gone on in my career, I've started maying more attention to who gets what lab upgrade funded, or what people get with their start-up packages, or what they negotiate for when they have leverage. Right now the flavor of the day seems to be multi-collector noble gas magnetic sector mass spectrometers; these allow for the simultaneous measurement of all of the different isotopes you need to measure for whatever technique you are involved in, thereby cutting down the uncertainty and time lags of changing magnet power, yada yada yada. I won't get started on that.
What I do want to talk about is step 2, sample collection and preparation. In particular I want to talk about turning a rock into an individual mineral, a process called mineral separation. Mineral separation fascinates me, but what really amazes me is how many people either ignore or do not understand the process.
Here is the problem: for almost all analyses you need to analyze pure mineral samples. Techniques which work on small single crystals (fission-track, (U-Th)/He, U-Pb) typically require minerals that are small (100-200 microns in length) and not overly abundant in the average granite (maybe form a 5 kg sample I'll get a few milligrams of apatite). Techniques that work on multiple crystals (biotite, muscovite, and k-spar Ar/Ar), typically require a few milligrams of very pure separates. They both, therefore, require methods of separating a rock into piles of individual minerals. Mineral separation is a blanket term that describes the various ways to turn a rock into a sample. The first step is almost always reducing the rock into individual mineral grains. This is typically done by crushing and grinding the sample, trying to get the minerals to break apart along grain boundaries.
In my experience, the next step is to run your sample over a rogers or gemeni table. These are basically large gold pans that concentrate the denser minerals (apatite and zircon in particular) into a smaller pile. This is then washed and dried, and run through a magnetic separator, basically a large magnet where you can vary the power and separate minerals based on their magnetic susceptibility. This is done is a series of steps, and a skilled mineral separator can obtain almost pure concentrates of the various "magnetic minerals" such as biotite, hornblende, and monazite. When you are done, you are left with a pile of non-magnetic mineral grains, including apatite and zircon.
If you need to get apatite and zircon, you must then enter the world of heavy liquids. Heavy liquids are exactly what they sound like, liquids with very high densities, anywhere from water (1.0 g/cm3) to 4.4 g/cm3. Because minerals have fairly specific densities, they will either sink or float in different heavy liquids. Zircon is very dense (4.6-4.7 g/cm3), and will sink in a liquid like MEI (Methylene Iodide density=3.33 g/cm3), while apatite (density 3.2 g/cm3) will float. Heavy liquids have been used in geology for a long time, but the particular liquids and their methods of use have changed significantly. Many of these liquids are toxic, and therefore kind of a pain to work with. Two of the nastier liquids I have fortunately never worked with, those are Clerici's Solution (Thallium Malonate density=4.36 g/cm3) and Bromoform (Tribromoethane, density=2.89 g/cm3). Clerici's Solution and Bromoform are not all that common anymore, mainly because there are now less toxic alternatives. TBE (tetrabromoethane density=2.95 g/cm3) is also fairly nasty, but is still in use in many labs, primarily because it has a lower viscosity than its non toxic alternatives SPT or LMT (Sodium Polytungstate or Lithium Metatungstate, density 2.85 g/cm3).
I was lucky in my graduate education to learn from one of the masters of mineral separation. While many people have used the same techniques and materials they learned on decades ago, my min sep teacher has continually improved and refined his techniques, trying as best as possible to increase cleanliness, and efficiency, and reduce unnecessary exposure to toxic liquids. He tells me he will soon have a web resource of his methods available, which will be advertised heavily on this blog. I am presently trying to implement some of his methods in my new lab. This is the first time I have had to work with TBE, or with large quantities of MEI, both of which, in my opinion, are completely avoidable.
But what fascinates me is how little attention this necessary step in thermochronology, or geochronology, typically receives. Would anyone dream of asking NSF Facilities for money to upgrade a mineral separation lab? The amount of time and money wasted in mineral separtion is really astonishing. I think though, that one of the reasons these facilities rarely receive the attention they deserve has to do with the hierarchy of the average thermochronology lab. One of the first jobs you delegate with increasing seniority is mineral separation. Right now we have a fleet of undergrads working for us helping crush, grind, and separate minerals. The drive to streamline the procedures is reduced when those of us in charge no longer have to do them. My main reason for trying to improve the set up is primarily because I don't like the idea of an 18 or 19 year old handling liters of TBE on my behalf, especially when there are good alternatives. Although old ways die hard, I think I have convinced a critical mass to support my efforts, and was even able to put in an order with our glassblower this week.
Aside from heavy liquids, I think the most exciting (albeit expensive) recent advancement in the art of mineral separations is the introduction of commercially available electric pulse disaggregators (EPD's). Instead of physically crushing and grinding rocks, EPD's send a pulse of electricity through your rock, which causes minerals to break apart along grain boundaries. The technique has the advantage of retrieving the crystals from the rock intact, that is, you don't run the risk of physically breaking them apart (a huge advantage for separating apatite.) The method was developed originally to work with small and relatively expensive lunar samples, but is amazing in what it can retrieve from a rock. The link above includes a movie showing how quick and easy the process can be. Although it would increase the quality and throughput of samples, they right now are pretty pricey (well, from a geologists point of view, from a college athletics perspective it would cost about 0.33 D1 college football coach yearly salaries, and in my current situation would have only 2 fewer wins.)
I believe wholeheartedly in the garbage in, garbage out philosophy. This is one reason that I think a great deal about mineral separation. I think when it comes to bang for the buck, this could be one of the best ways to improve lab productivity. I'd be interested in other people's experiences with mineral separation, especially if you have used something safer or less toxic that the heavy liquids I described.
Friday, July 27, 2007
Credit for e-cademic work
He pursued the issue with the publisher and got responses from both the publisher and one of the authors. The publisher responded with two legal questions, first whether or not a copyright could be asserted for a web site, and second if a bibliography could itself be copyrighted, since "presumably all bibliographies are compilations of previous bibliographies." I don't like either of these questions myself, although admittedly all of my legal training is from "Law and Order" episodes, and to my knowledge they haven't yet tackled a case like this. Whether or not it is legally copyrighted is besides the point, it goes straight to the heart of academic honesty. How many times do you see personal communications referenced in papers? Especially when the product is something you are hoping to sell and make money off of, the legality should be secondary. The second point the publisher makes, about whether or not bibliographies can be copyrighted at all, makes me want to retype their book, print it off at Kinkos and sell it for half price, I bet we'd then see how copyrighted they think bibliographies can be.
The author responded with an apology and explanation that in the rush to finish and publish all reference to the web site or its creator was accidentally omitted. This excuse, to me, is lame. Perhaps it could be accepted if it was one reference or some small little factoid, but from the description in the article the web site represented a tremendous amount of work. That is academic dishonesty, plain and simple; either that or the book author is a complete buffoon and/or donkey.
But what protection does any of our original work have? I've never worried about it, this is not something I am doing for money, and I have yet to publish anything that I'd consider a serious academic contribution. I also don't care if people use anything I do on a not-for-profit basis, that is kind of the whole point. Other bloggers have certainly done more original work (or are aiming to do more) and although they see this as an open way to provide quality earth science educational materials, I am not sure anyone would be happy seeing their work copied in a textbook, especially one of those $125.00 gems intro students now have to pony up for. I suppose the hope is that since we provide the information free of charge that there is no incentive to copy our e-cademic work, but are there really any protections? Will our web sites become open farms for textbook authors with fast approaching deadlines?
Thursday, April 12, 2007
Wunderground and free public data
We in the American northeast have been enjoying a pretty late winter this year, meaning, it's a hell of a lot colder than April is supposed to be. The place I used to live was, in general, much warmer, and I got to thinking of the temperature difference between where I used to live. This got me hitting Wunderground, a weather site I started checking thanks to my Mom (an amateur weather guru who often knows more about my weather from 3000 miles away than I do). One of the cool things about Wunderground is that they make it easy to download historical weather data. For example, after about 10 minutes of clicking and Excelling I made this
 Which shows the temperature difference between where I lived in grad school and where I live now. The y-axis is the difference in average daily temperature (°F) between old home and new home; values above 0 mean it is warmer at BWRU, and values below 0 mean it is warmer here at ESRU. The line is a five day running average. Why 5 days? I have no idea, but I'd appreciate a good reason if anyone can think of one. There are a couple things I noticed after putting this together. First, we had a really warm winter here at ESRU, until well into January it really wasn't much warmer back at BWRU. Second, there is an odd apparent periodicity in the running average, with a fortnightly recurrence. You can sum up my statistical analysis of this feature with the words "Jack Squat," but it is still potentially interesting. The maximum difference was just a month ago, March 6th when it was 59° colder here than there.
Which shows the temperature difference between where I lived in grad school and where I live now. The y-axis is the difference in average daily temperature (°F) between old home and new home; values above 0 mean it is warmer at BWRU, and values below 0 mean it is warmer here at ESRU. The line is a five day running average. Why 5 days? I have no idea, but I'd appreciate a good reason if anyone can think of one. There are a couple things I noticed after putting this together. First, we had a really warm winter here at ESRU, until well into January it really wasn't much warmer back at BWRU. Second, there is an odd apparent periodicity in the running average, with a fortnightly recurrence. You can sum up my statistical analysis of this feature with the words "Jack Squat," but it is still potentially interesting. The maximum difference was just a month ago, March 6th when it was 59° colder here than there.OK, great, it is colder in the northeast than a western school at a lower latitude, big shocker. Doing this though reminded me of an exercise I came up with when teaching an earth hazards course in 2004, again using Wunderground. In February of 2004 there was flash flooding in the southern part of San Francisco. Pretty exciting, there was water rushing down stairwells and elevator shafts at San Francisco State University. At this time I was teaching this earth hazards course at an area school, so I used Wunderground to come up with this homework. I had the students get the rainfall data for two days from the same station, the flash flood day (2/25/2004) and another day, a few months earlier that had some serious rainfall but resulted in no flash flooding (12/29/2003). This is the graph they were supposed to come up with

The y-axis is rainfall rate (in inches per hour), and the x-axis is time. The total rainfall for both days was actually remarkably similar, actually 12/29 had more cumulative rainfall, but the flash flood day had all that rain packed into less than an hour, instead of spread out over the day. Basically I was driving home the point that flash floods, unlike regional floods, come from large amounts of rain in small amounts of time.
What both of these exercises got me thinking about is free public data sets. Wunderground does not provide the kind of really rigorous data I'd use if I was working on an actual publishable study, but as a teaching tool or means of illustrating basic earth science concepts, it is fantastic. So Wunderground is great for relatively recent weather data. What about other public earth science related data repositories?
Wednesday, April 11, 2007
More on Reviews
Recently Lab Lemming started a good discussion on some of the issues involved with scientific reviews, specifically reviewer anonymity. This, and related posts throughout the geoblogosphere were especially timely for me. In the past two weeks I have reached two milestones in my scientific career. First, I wrote and submitted my first official review of a paper submitted to an honest to god reputable scientific journal. I was pretty excited about this to begin with. I have heard many of my bosses and older sciencey folk talk in exasperated tones about all of the papers they have to review and what a pain it was. I suppose after I've done this a hundred times I'll be sick of it, but for now it made me feel like a real professional geologist person. Anyways, I spent a lot of time on this review, wrote and re-wrote my comments, slept on it, read the paper a fourth time, and finally sent in the final comments. In general I was positive in my review, although I did raise some excellent points, which I hope to be thanked for generously in the acknowledgements paragraph of the final paper. How will I be thanked? Well, as an anonymous reviewer of course. This was a tough call for me. Theoretically I do not like anonymous reviews. I stand behind my comments and evaulation, so why don't I attach my name to them? I wasn't overly harsh or negative, and I even pointed out lots of good things. But why do I still fear retribution because I recommended acceptance only with revisions? Truth is, I think as a post-doc who will in the not too distant future be on the job market, I am not sure how much I want to risk souring potential search committee member's opinions of me. I do not yet have the position or name recognition to feel insulated at all from negative vibes. Even though I know I gave a fair and honest assessment, I left my name off the final review. Maybe Thermochronic is just a big old chicken?
And, let's add smarmy hypocrite to big old chicken, because I have also spent the past week grumbling about the second milestone of the past two weeks that I have achieved, namely getting an off-the-mark and anonymous negative review of a paper. This is not a paper I am first author on, but that has not stopped me from cursing anonymous reviewer #n under my breath. I don't know if anonymity played much of a role in making this review crappy, but I would at least like the name of a geologist to google and curse at while I take breaks from revisions. What kills me about this review is that (1) all of the other reviews were very positive, and (2) the comments actually didn't raise any significant issues, just the reviewer's unfamiliarity with some of our standard laboratory techniques.
I like the suggestion of Lab Lemming to include signed copies of reviews with tenure packages. I actually think we could take this a little farther and make reviewer anonymity temporary. If a paper gets published, and a year later the names of the reviewers are made available, then as long as the reviews were honest I think many of the fears of pay-back would be gone. Right now I am all hopped up, stomping around when I think of anonymous reviewer #n and his/her goofy comments, but when the paper is published, a year from now, the emotion of the rejection would be largely gone, and I'd be able to see the review in a much clearer light. Come to think of it, as I sit at the "Scientific Paper of the Year" awards banquet with my co-authors we will probably laugh about the whole incident. So, how's that, a statute of limitations on reviewer anonymity.
Wednesday, March 14, 2007
Global Climate Energy Project, Big Oil, and Big Benefactors
“Exxon Mobil is trying to greenwash itself, and it’s using Stanford as its brush,” Yusef Robb, who has worked with Bing on climate issues, told The Mercury News. “We think that people who give to Stanford do so because they want to help the future leaders of this nation, not because they want to advance the agenda of ExxonMobil.”
I am by no means a fan of ExxonMobil in the political or environmental sense, I am sure they, like most any other company in the world, make decisions based on short term profits and not on some sun-shiney moralistic ideal. But I think Bing's logic is twisted for a few big reasons.
- I don't see the problem with a company trying to "greenwash" it's image. I don't believe companies can be moral or immoral, they can just do good things or bad things. ExxonMobil has plenty of bad company points, as I have already blogged about (and then some for sure), but why should that stop them from doing something good? Giving money to research aimed at developing energy alternatives seems like a good thing (as long as there are no strings attached, see point #3.)
- I am sure the Bing family has in the past had plenty of money invested with oil companies (perhaps without them even knowing about it.) Most banks and mutual funds and things of that sort do. So why would it be OK to have investments that use companies like ExxonMobil to make money, but not want anyone else to benefit. Bing could of course only have "socially responsible" investments. I don't know all that much about him, other than what I can find on imdb.com and related entertainment sites, so if Mr. Bing does only have socially responsible investments, flies commercial airlines (so as not to waste energy with private planes), takes public transportation as often as possible and drives a hybrid when he can't, lives in a modest home complete with native fauna in the garden and of course, no swimming pool (I am guessing he lives in Southern California since he is a Hollywood dude), a nice compost pile where he can put the scraps from his locally grown vegetarian cooking, then I will perhaps agree with his decision.
- This institute could be very important. It makes sense that energy companies want to invest in the future of energy, but isn't this something we all have a vested interest in. It states explicitly that Stanford University will own any and all patents produced by the institute, so they won't directly make money from this.
- The gift he is rescinding was a general gift to the university; the gift he is complaining about is building a new institute. So, the general student population will be hurt, but those involved in the institute won't.
- I think oil companies are easy targets to complain about. Truth is, like almost all large companies, they do some pretty bad stuff. I have no problem passing laws that regulate oil companies or the use and/or acquisition of energy, but I also think that the first place we need to look is at our energy use. If there wasn't such an absurd demand for oil, then oil companies would just be another podunk company, it is our use that gives them the power they have. Another example, I think, is Ted Turner. I again agree with him on most things, but he flies around the world in a private jet, perhaps the most energy inefficient mode of transportation known to man (well, except for perhaps spotted owl powered space shuttles or other monstrosities). Fly first class if you must, mingle with the dregs of society and enjoy the average in-flight movie; a small price to pay for your planet. Hell, they could even afford those super sweet Bose noise cancelling headphones, those would make even coach seats bearable. This kind of reminds me of people who drive SUV's to Whole Foods so they can buy organic arugula.
- Stanford, I am sure, has had investments in ExxonMobil for a long time, as does anyone, or anything, with a diversified investment portfolio (see point #1). Why is it an issue now? Why doesn't Bing work with Stanford to create a socially responsible investment portfolio, or encourage Stanford to use it's shareholder clout to help redirect wayward corporations?
- Let's pretend that every major oil company in the world except ExxonMobil had contributed to the institute, my bet is that people would then complain about how ExxonMobil isn't doing enough to help find energy alternatives.
- Does his action help solve a problem, or is it simply a publicity stunt? If the end goal is to reduce our dependence on fossil fuels and reduce the production of greenhouse gases, does this help, hinder, or do nothing? Would he rather take away $100 million dollars from the institute, would that solve the problem?
I have no problem with Mr. Bing's positions or politics, I just think this is an odd reaction. Perhaps he could direct the gift into something that directly counteracts what he dislikes about ExxonMobil. Maybe create his own institute, one that develops oil company free investment plans. This isn't the first time a major benefactor has broken a promise of support to Stanford, and I don't think in either case the decision made much sense.
I am, however, excited to see that Steve Bing is producing a film version of Beowulf, which ranks as my #1 all time solo--road- trip book-on-tape. It got me to and from Seattle many times.
Friday, March 09, 2007
Peak Oil and Biophysical Economics
The figures I am using are all from the wikipedia Peak Oil page, just a disclaimer
Peak Oil is an idea first developed by one of this century's great geologists, M. King Hubbert. He introduced his theory in the late 1950's as a way of predicting future oil supplies. The idea is fairly simple, and is based on the fact that oil is a non-renewable resource, and there is consequently a finite supply of oil in the world. Oil production will follow a bell shaped curve. As the demand for oil increases, oil companies increase their supply as much as they can; pumping fields at full capacity and discovering new reserves. Demand continues to increase, but fields begin to dry up, and new fields just don't exist, so production declines. Whatever the exact shape of the curve, the logic is pretty plain, the integrated area under the curve is equal to the total amount of recoverable petroleum reserves in the world. This is Peak Oil, the idea that there will (or has been) a peak in oil production, and that at some point production will decrease, about as rapidly as it initially increased. This model works very well for individual oil fields, or even national reserves
As the demand for oil increases, oil companies increase their supply as much as they can; pumping fields at full capacity and discovering new reserves. Demand continues to increase, but fields begin to dry up, and new fields just don't exist, so production declines. Whatever the exact shape of the curve, the logic is pretty plain, the integrated area under the curve is equal to the total amount of recoverable petroleum reserves in the world. This is Peak Oil, the idea that there will (or has been) a peak in oil production, and that at some point production will decrease, about as rapidly as it initially increased. This model works very well for individual oil fields, or even national reserves
The red curve shows US oil production versus time, with a peak in 1970, and a slight secondary bump thanks to the north slope of Alaska. World oil is in blue.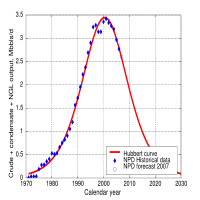
This is the theoretical production curve (red) and actual data for Norwegian production. I don't want to argue the specifics of the model; whether or not the curves are symmetric, how much technology can help (answer jack squat), when the peak is, etc.. There are some truths that you just can't get around, namely that the amount of oil in the world is finite. and that at some point we will be using more than we can extract, and production rates will decline.
Arguments against peak oil seem to follow the lines of "the market solves everything." As supply decreases, price increases, and demand decreases, thank god for the market, it will save us all. Oil will get expensive, and some budding entrepreneur will devise some amazing technology that will save the world! At some level I suppose that is true; if there are humans around in 500 years, they will undoubtedly not be using oil as their primary energy source (they will of course be using flux capacitors). Whether or not it happens isn't the problem, but instead what happens during the transition. Dismissing peak oil with some dreamy ode to market forces ignores how nasty things can get in the face of scarce resources. Technology will find alternatives, but when? Do we decide now to solve the problem before it gets too hairy, or do we wait until something resembling this happens? Hell, remember when stores would run out of Beanie Babies and all hell would break lose? I think oil might be worse.
This brings me to biophysical economics. The term, as used by Charlie Hall, refers to an economic theory that does not violate the laws of nature, especially trivial little ideas like thermodynamics. This is of course a major problem with simple supply and demand, it assumes that supply is only limited by something like factory output. For example, no matter how much the price of oil increases, the supply cannot, and any reasonable replacement would take much longer to develop than the oil would last. Economic theory rooted in physical realities, I like it.
I am less an economist than I am a petroleum geologist (not much), so this post is really not in my wheelhouse. This has sparked my interest though. Are there any more econ-minded folks out there who know about biophysical economics, or any economic theory that explicity takes into account the laws of nature? Any notable economists who consider the natural world, or economics programs that require some line of study like this?
Wednesday, January 31, 2007
San Francisco, the city that waits to die!



The only part of the movie that I still think is a little absurd is the tone with which it is narrated (by Paul Vaughan). Basically, Americans are idiots, look how stupid they are, man, no one else in the world is as stupid as those bloody Americans. Although the tone is kind of annoying, what gets me about this film is that I can imagine, 30 years from now, watching something similar but this time dealing with global warming. Scientist after scientist is trotted out and basically says "we know it is dangerous, we know we put buildings where we shouldn't, we know many of the buildings aren't safe, but there isn't much we can do about it." Politics and real estate dollars rule. Some notables that make appearances include
Louis C. Pakiser, the first director of the U.S.G.S. earthquake center at Menlo Park and 1996 recipient of the Geological Society of America Distinguished Service Award

CalTech Geophysicist Clarence Allen

Geothechnical engineer, Cal Professor, and alleged outstanding soccer player Harry Seed. Here he is demonatrating liquefaction. If you look closely, you can see a small yellow house perched on a column of wet sand, that is then shook until the house sinks. Incidentaly, this house is roughly the same size as my last apartment in California.

CalTech Professor Emeritus George Housner, one of the original earthquake engineers (distinct from the O.G.'s)

David Evans, one of, if not the first person to demonstrate the relationship between pore fluid pressure and induced seismicity (see Evans, D.M. (1969), Fluid Pressure and Earthquakes, Eos, Transaction, Amer. Geophys. Union, 50, 5, p. 387-388.)

And who demonstrates the famous beer can experiment

and Darrell Wood (sp?), sitting in front of "one of the largest computers in the world," at Stanford's linear accelerator.

There are a few fantastic statements about earthquake prediction, guaranteed to bring some chuckles, including, "if the [next] San Francisco earthquake does not occur within the next 5 years it is my opinion that we will be able to predict it." I remember actually being kind of struck by some of footage. I remember especially the discussion of the numerous buildings, including schools, hospitals, and police stations, that sit right above the Hayward fault. Like the home of the second best college football team in the bay area (below)

Or my favorite, an old school for the deaf in Berkeley, which is now the Clark Kerr campus (where at the time I first saw this film I had a friend living)

Also footage of the creeping part of the fault system in Hollister

And footage of a USGS led project to monitor slip across a strand of the fault by using geodolites. The footage below is from Mount Diablo where they were making measurements


Perhaps the oddest thing though is the soundtrack for the movie. It is "California Earthquake" by Mama Cass. The full on song blares in the beginning and end of the movie, when the motorcycles are cruising all over the place, over the bay bridge, through the streets of the city, catching major air... I am not sure what the connection is between the motorcycles, Mama Cass, and earthquakes. During the more reflective parts of the film, we are treated with a tuned down instrumental version of the song. Truth is, without this soundtrack, I don't think the movies would really have stuck in my head, it would have just been a normal old boring class film strip. Well, except for the predictions about predicting earthquakes and the narrators apparent excitement about the prospect of using nuclear weapons to trigger earthquakes. Fortunately none of the geologists interviewed supported the idea of intentionally triggering earthquakes with H-bombs.

This movie was released in 1971, filmed in 1970. This is really at the beginning of the acceptance of plate tectonics as a field. Tectonics is referred to at a few points in the film, mainly the ideas of plates, but it certainly doesn't play a large role, and there are some definite factual errors. But really, for the state of the field, the information isn't really that bad. I also am thankful that no one is asking me on tape to make predictions, because my track record....well, err..
Prof. Advisor "Hey Thermo, when is this alleged lab you've been building going to be up and running?"
PhD Candidate Thermochronic, "Well, assuming that turbo pump is ready for me to pick up Friday, I'll be pumping out data by spring of 2002, easy."
Is this movie standard fare at other schools? I see it in many library holdings, small liberal arts school had a copy, is it pervasive? Am I crazy to think with a better editor and less annoying soundtrack this movie might be excellent?
Wednesday, December 27, 2006
The Racetrack, Death Valley, California

 So, Many of you will recognize these. Well, when I say many of you, I mean me, who is the only person really reading this blog. But, just in case you stumbled across this page, and know the answer to my question......the Racetrack, in Death Valley, these immense rocks "mysteriously" move across one end of a playa. Allegedly, no one has ever seen these rocks move. They can be quite large, much heavier than even I, with my superhuman sterngth, could not move. I have seen other geologists present theories on how they move, and anyone who has walked on wet playa mud or been in Death Valley during heavy winds can imagine them being blown all over the place. My question is, are there other places in the world with the same "moving rocks?" Anyone? Thanks.
So, Many of you will recognize these. Well, when I say many of you, I mean me, who is the only person really reading this blog. But, just in case you stumbled across this page, and know the answer to my question......the Racetrack, in Death Valley, these immense rocks "mysteriously" move across one end of a playa. Allegedly, no one has ever seen these rocks move. They can be quite large, much heavier than even I, with my superhuman sterngth, could not move. I have seen other geologists present theories on how they move, and anyone who has walked on wet playa mud or been in Death Valley during heavy winds can imagine them being blown all over the place. My question is, are there other places in the world with the same "moving rocks?" Anyone? Thanks.

